
Chemistry, 29.03.2021 18:10 yugbug44owwc7w
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much energy is required?
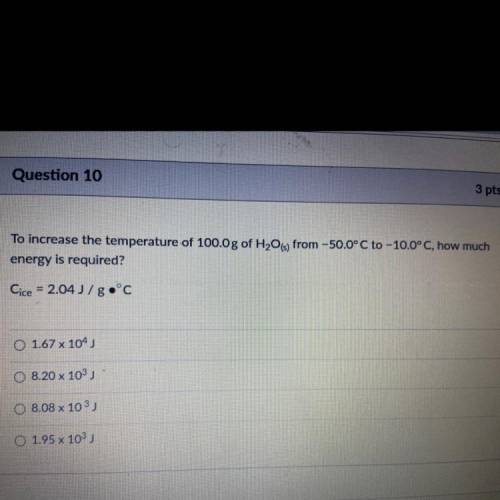

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 23.06.2019 15:30
Among these processes, which is the slowest chemical reaction? a. digesting food b. boiling an egg c. tarnishing of silver d. melting of a glacier
Answers: 2

Chemistry, 23.06.2019 15:40
Sugar is made up of clear, colorless crystals that dissolve easily in water, but the crystals and their solution do not conduct electricity. which statement describes sugar? it is made up of atoms that are held together by metallic bonds. it is made up of atoms that are held together by covalent bonds, it is made up of atoms that are held together by weak ionic bonds. it is made up of atoms that are held together by strong ionic bonds.
Answers: 1
You know the right answer?
To increase the temperature of 100.0 g of H2O(s) from -50.0°C to -10.0°C, how much
energy is requir...
Questions

Mathematics, 13.10.2021 02:40

History, 13.10.2021 02:40

Computers and Technology, 13.10.2021 02:40

Physics, 13.10.2021 02:40

Mathematics, 13.10.2021 02:40





Mathematics, 13.10.2021 02:40






Mathematics, 13.10.2021 02:40

Computers and Technology, 13.10.2021 02:40



Social Studies, 13.10.2021 02:40



