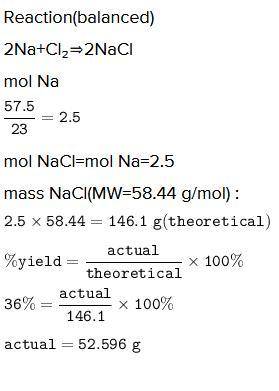Chemistry, 29.03.2021 19:20 joyceslater16
A chemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. The chemical reaction that occurred is shown.
Na + Cl2 → NaCl
If the percentage yield of the reaction is 86%, what is the actual yield? Show your work, including the use of stoichiometric calculations and conversion factors.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
A chemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. The chemical re...
Questions










English, 02.09.2020 04:01



Mathematics, 02.09.2020 04:01

English, 02.09.2020 04:01

Mathematics, 02.09.2020 04:01

Chemistry, 02.09.2020 04:01



Mathematics, 02.09.2020 04:01

Mathematics, 02.09.2020 04:01