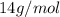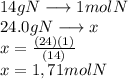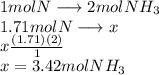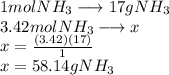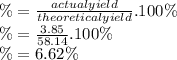Chemistry, 26.08.2019 11:20 ammullims822
A24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 3.85 g nh3. what is the percent yield for this reaction given the reaction: n2(
g. 3h2(
g. --> 2nh3(g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
A24.0 g sample of nitrogen gas reacts with an excess of hydrogen gas to give an actual yield of 3.85...
Questions

Social Studies, 16.04.2021 03:10

Mathematics, 16.04.2021 03:10




Chemistry, 16.04.2021 03:10


Mathematics, 16.04.2021 03:10



English, 16.04.2021 03:10

Mathematics, 16.04.2021 03:10

History, 16.04.2021 03:10







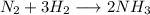
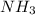 is obtained
is obtained