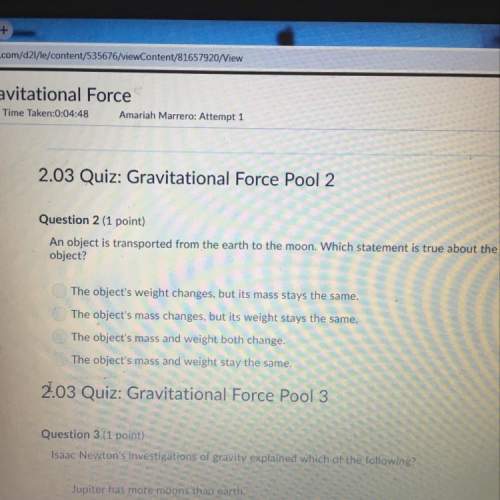
Chemistry, 29.03.2021 23:30 lovenot1977
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
The table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory.
Initial Mass and Yield
Sulfuric Acid Aluminum Hydroxide
Initial Amount of Reactant 35 g 30 g
Theoretical Yield of Water from Reactant 12.85 g 31.77 g
What is the approximate amount of the leftover reactant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
The following reaction shows the products when sulfuric acid and aluminum hydroxide react.
2Al(OH)3...
Questions

Mathematics, 01.08.2020 02:01







Mathematics, 01.08.2020 02:01



English, 01.08.2020 02:01




English, 01.08.2020 02:01





Mathematics, 01.08.2020 02:01