
Chemistry, 29.03.2021 23:50 ahmadfarriz4088
The equilibrium constant, Kc, for the reaction below is 0.10 at 25oC. Find the equilibrium concentration of chlorine gas, Cl2(g), if the equilibrium concentrations of ICl(g) and I2(g) are known to be 0.50 M and 0.40 M respectively. 2 ICl(g) → Cl2(g) + I2(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

You know the right answer?
The equilibrium constant, Kc, for the reaction below is 0.10 at 25oC. Find the equilibrium concentra...
Questions

History, 26.11.2019 23:31

History, 26.11.2019 23:31

Mathematics, 26.11.2019 23:31



Mathematics, 26.11.2019 23:31

Chemistry, 26.11.2019 23:31

Chemistry, 26.11.2019 23:31

History, 26.11.2019 23:31


Health, 26.11.2019 23:31


Mathematics, 26.11.2019 23:31

Mathematics, 26.11.2019 23:31



Chemistry, 26.11.2019 23:31

History, 26.11.2019 23:31

Chemistry, 26.11.2019 23:31

Spanish, 26.11.2019 23:31

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b}}](/tpl/images/1228/6809/b30b0.png)
![Kc=\frac{[Cl_{2} ]*[I_{2} ]}{[ICl]^{2} }](/tpl/images/1228/6809/84847.png)
![0.1=\frac{[Cl_{2} ]*0.40 M}{(0.50 M)^{2} }](/tpl/images/1228/6809/27c5a.png)
![0.1=\frac{[Cl_{2} ]*0.40 M}{0.25 M^{2} }](/tpl/images/1228/6809/23319.png)
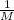 * [Cl₂]
* [Cl₂]


