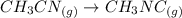Chemistry, 30.03.2021 05:30 valenciafaithtorres
g At the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reaction vessel. After 25 min, 0.111 mol of reactant (CH3NC) remain. There are mol of product (CH3CN) in the reaction vessel. At the start of an experiment, there are 0.200 of reactant and 0 of product in the reaction vessel. After 25 , 0.111 of reactant remain. There are of product in the reaction vessel. 0.022 0.089 0.311 0.111 0.200

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1


Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
g At the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reactio...
Questions



English, 22.11.2020 15:40




English, 22.11.2020 15:40

History, 22.11.2020 15:40



Spanish, 22.11.2020 15:40



Mathematics, 22.11.2020 15:40


English, 22.11.2020 15:40


Social Studies, 22.11.2020 15:40

Physics, 22.11.2020 15:40

Physics, 22.11.2020 15:40