Chemistry, 30.03.2021 14:00 cxttiemsp021
What is the mass of 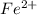 in 5 tablets of iron if the number of moles of
in 5 tablets of iron if the number of moles of 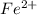 is 4.2225 x
is 4.2225 x 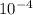 mol.
[ Ar = Fe, 56 ]
mol.
[ Ar = Fe, 56 ]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
What is the mass of in 5 tablets of iron if the number of moles of is 4.2225 x mol.
[ Ar = Fe, 5...
Questions

English, 19.11.2020 23:40

Social Studies, 19.11.2020 23:40

History, 19.11.2020 23:40



Mathematics, 19.11.2020 23:40

Social Studies, 19.11.2020 23:40



Health, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Spanish, 19.11.2020 23:40

Health, 19.11.2020 23:40

Computers and Technology, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

History, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Biology, 19.11.2020 23:40

English, 19.11.2020 23:40

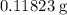 in total for the five tablets.
in total for the five tablets.  per tablet.)
per tablet.)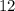 atom.
atom. 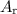 of iron is
of iron is 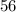 . In other words, the mass of one mole of iron
. In other words, the mass of one mole of iron 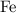 atoms would be approximately
atoms would be approximately 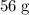 .
.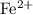 ions. Each
ions. Each 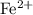 ion contains two fewer electrons than a neutral
ion contains two fewer electrons than a neutral 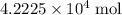 of
of 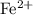 ions might be lighter than the same number of
ions might be lighter than the same number of 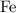 atoms by a very small extent: The mass of one mole of electrons is approximately
atoms by a very small extent: The mass of one mole of electrons is approximately 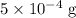 , much smaller than the mass of the same number of
, much smaller than the mass of the same number of 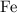 atoms (approximately
atoms (approximately 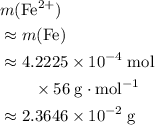 .
.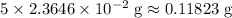 of
of