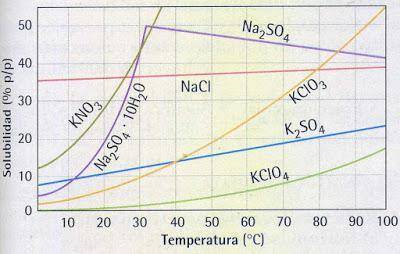
1. Analyze the graph and solve the following
questions
a) What is the substance with the...

Chemistry, 30.03.2021 20:20 AlaishaWiseBrown
1. Analyze the graph and solve the following
questions
a) What is the substance with the greatest variation
solubility? justify your answer
b) How is the solubility of KClO4 compared to the
NaCl solubility?
c) At 50 ºC, what is the solubility of KCLO3?
d) At 20 ºC, which is the substance with the lowest
solubility?
e) At 60 ºC, what is the solubility of Na2SO4?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Questions


English, 22.08.2019 00:30



Biology, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30


Social Studies, 22.08.2019 00:30

History, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30


History, 22.08.2019 00:30





Mathematics, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30



