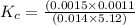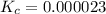Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) + H2O(I) rightarrow F-(aq) + H3O+(aq)
At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition:
Compound Amount
HF 1.62 g
H2O 516 g
F- 0.163 g
H3O+ 0.110 g
Calculate the value of the equilibrium constant for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) +...
Questions

Health, 27.01.2020 15:31





Mathematics, 27.01.2020 15:31


Chemistry, 27.01.2020 15:31


Mathematics, 27.01.2020 15:31

History, 27.01.2020 15:31

Mathematics, 27.01.2020 15:31

Mathematics, 27.01.2020 15:31


Mathematics, 27.01.2020 15:31


History, 27.01.2020 15:31


Mathematics, 27.01.2020 15:31

Mathematics, 27.01.2020 15:31

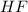 =
= 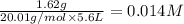
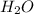 =
= 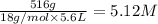
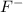 =
= 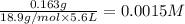
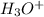 =
= 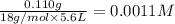
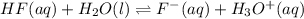
![K_c=\frac{[F^-]\times [H_3O^+]}{[HF]\times [H_2O]}](/tpl/images/1230/8174/06b96.png)