2 attempts left
Check my work
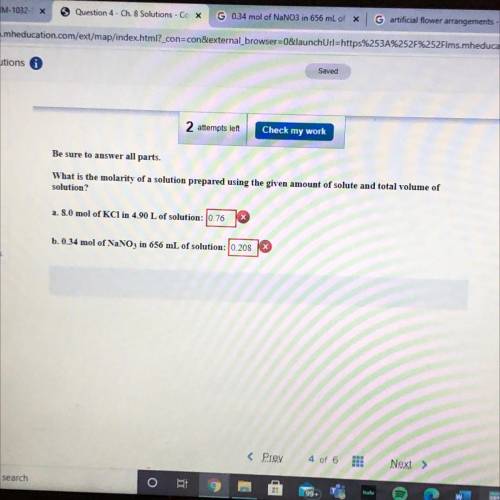
Be sure to answer all parts.
What is the molarity of a so...

Chemistry, 30.03.2021 23:30 princesstn28oqlfir
2 attempts left
Check my work
Be sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 8.0 mol of KCl in 4.90 L of solution: 0.76
X
b. 0.34 mol of NaNO3 in 656 mL of solution: 10.208
X

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1


Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Questions



Mathematics, 23.05.2020 22:01

Mathematics, 23.05.2020 22:01

English, 23.05.2020 22:01










Law, 23.05.2020 22:01

Computers and Technology, 23.05.2020 22:01






