
Chemistry, 31.03.2021 09:00 uberagentkenny
What is the hydrogen ion concentration in molarity if the hydroxide ion concentration is 7.609 10-13 ?
[H+][OH-] = 10-14
pH = -log[H+]
[H] = 10pH

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

You know the right answer?
What is the hydrogen ion concentration in molarity if the hydroxide ion concentration is 7.609 10-13...
Questions

English, 11.04.2020 03:43


Computers and Technology, 11.04.2020 03:43

Mathematics, 11.04.2020 03:43

Mathematics, 11.04.2020 03:43


Mathematics, 11.04.2020 03:43


Mathematics, 11.04.2020 03:43

English, 11.04.2020 03:43




Chemistry, 11.04.2020 03:43






![pOH=-\log[7.609\times 10^{-13}]](/tpl/images/1232/0218/fc707.png)
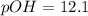
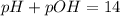
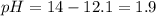
![pH=-\log [H^+]](/tpl/images/1232/0218/37e81.png)
![[H^+]=0.013M](/tpl/images/1232/0218/4829c.png)


