ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the m...

ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the minutes go faster than actual minutes.
The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K.
Follow these steps, and then record your observations:
Locate the orange reset button on the bottom right side of the screen.
Press reset to start the reaction over.
Drag the top of the ruler upward until it reaches the 40 mark.
Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler.
Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready.
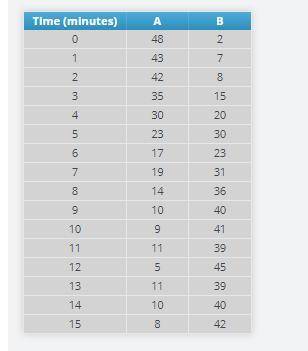
Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box.
At every minute on the timer, pause the simulation and record the number of A and B particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Questions

Social Studies, 27.05.2020 21:57



Mathematics, 27.05.2020 21:57




Mathematics, 27.05.2020 21:58

Mathematics, 27.05.2020 21:58

Mathematics, 27.05.2020 21:58






Chemistry, 27.05.2020 21:58

Computers and Technology, 27.05.2020 21:58

Mathematics, 27.05.2020 21:58





