
Chemistry, 31.03.2021 20:50 alexkrol10
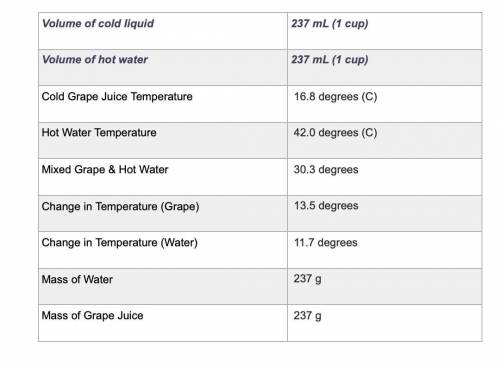
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the specific heat for the liquid you selected. Use the equation qwater = m × c × ΔT to calculate the heat lost by the hot water. Show your work using the problem-solving method shown in previous rubrics.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the speci...
Questions

Mathematics, 18.10.2019 10:10


History, 18.10.2019 10:10


Social Studies, 18.10.2019 10:10


Mathematics, 18.10.2019 10:10




Social Studies, 18.10.2019 10:10




Computers and Technology, 18.10.2019 10:10







