
Chemistry, 01.04.2021 01:00 romanlittlewood
ASAPPP HELP NO LINKS
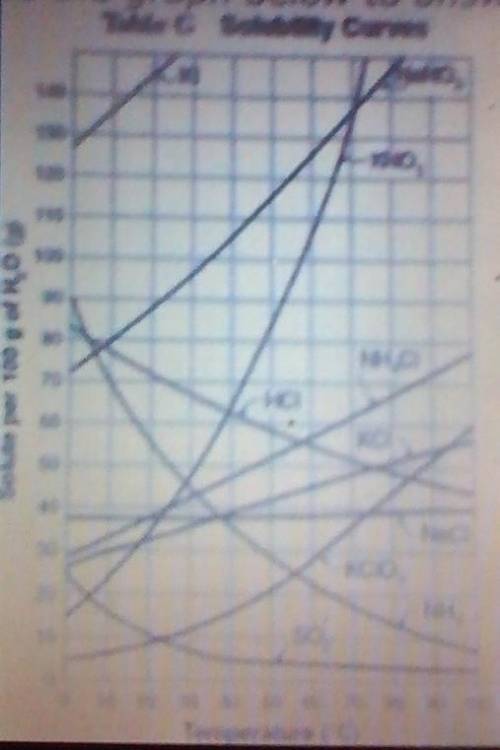
Ammonia (NH3) dissolved in water is heated in a beaker. If initially there had been 60 grams dissolved in 100 grams of water, what would happen if the temperature reached 90℃ and why? (AKS 6c DOK 2)
A.
The solution would become supersaturated and the ammonia would all remain dissolved .
B.
The solution would become saturated but the amount of ammonia dissolved would remain the same .
C.
about 50 more grams of ammonia would be able to dissolve as the solution has become unsaturated .
D.
about 50 grams of ammonia would come out of solution as the temperature caused the solubility to decrease .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
ASAPPP HELP NO LINKS
Ammonia (NH3) dissolved in water is heated in a beaker. If initially there had...
Questions

Mathematics, 05.05.2020 03:47





Mathematics, 05.05.2020 03:47

History, 05.05.2020 03:47

Mathematics, 05.05.2020 03:47




Mathematics, 05.05.2020 03:47








Biology, 05.05.2020 03:47



