
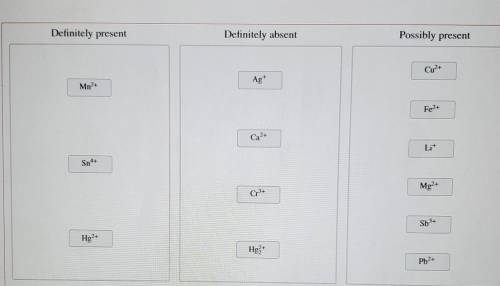
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate formed. Next, HS was bubbled through the acidic solution. A precipitate formed and was filtered off. Then, the pH was raised to about 8 and HS was again bubbled through the solution. A precipitate again formed and was filtered off. Finally, the solution was treated with a sodium carbonate solution, which resulted in no precipitation.
Classify the metal ions based on whether they were definitely present, definitely absent, or whether it is possible they were present in the original mixture.
Mn2+
Sn4+
Hg2+
Ag+
Ca2+
Cr3+
Hg2 2+
Cu2+
Fe2+
Li+
Mg2+
Sb3+
Pb2+

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2


Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
A solution containing a mixture of metal cations was treated with dilute HCI and no precipitate form...
Questions

English, 10.12.2020 05:30




Mathematics, 10.12.2020 05:30




Biology, 10.12.2020 05:30

Mathematics, 10.12.2020 05:30

Chemistry, 10.12.2020 05:30


Health, 10.12.2020 05:30

Business, 10.12.2020 05:30

English, 10.12.2020 05:30



English, 10.12.2020 05:30

Spanish, 10.12.2020 05:30

Mathematics, 10.12.2020 05:30



