
Chemistry, 01.04.2021 06:40 Knownothing
Calculate the pH of a solution formed by mixing 550.0 mL of 0.30 M HClO with 275.0 mL of 0.20 M CsClO. The Ka for HClO is 2.9 × 10-8.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Calculate the pH of a solution formed by mixing 550.0 mL of 0.30 M HClO with 275.0 mL of 0.20 M CsCl...
Questions




Mathematics, 23.10.2020 20:40


Chemistry, 23.10.2020 20:40

Mathematics, 23.10.2020 20:40



Biology, 23.10.2020 20:40

English, 23.10.2020 20:40


Mathematics, 23.10.2020 20:40

Mathematics, 23.10.2020 20:40



Mathematics, 23.10.2020 20:40

History, 23.10.2020 20:40

English, 23.10.2020 20:40

Mathematics, 23.10.2020 20:40

![\frac{[ClO^-]}{[HClO]}](/tpl/images/1234/3126/d9b8f.png)
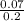 = 7.08
= 7.08

