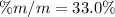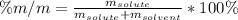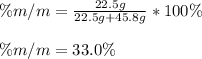Chemistry, 01.04.2021 07:00 katii54feliz
If we add 22.5 g of HCl to 45.8 g of water, what is the percent by mass of HCl in the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
If we add 22.5 g of HCl to 45.8 g of water, what is the percent by mass of HCl in the solution?...
Questions

Mathematics, 12.03.2021 14:00

Mathematics, 12.03.2021 14:00



English, 12.03.2021 14:00

Mathematics, 12.03.2021 14:00

Mathematics, 12.03.2021 14:00

English, 12.03.2021 14:00




Mathematics, 12.03.2021 14:00

English, 12.03.2021 14:00


Computers and Technology, 12.03.2021 14:00




Mathematics, 12.03.2021 14:00