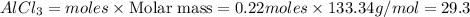PLEASE HELP
25.0g of Aluminum metal is combined with
45.Og Copper(ll) Chloride to produce Alu...

PLEASE HELP
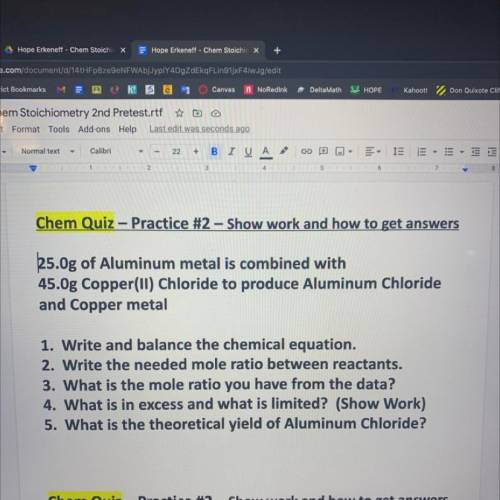
25.0g of Aluminum metal is combined with
45.Og Copper(ll) Chloride to produce Aluminum Chloride
and Copper metal
1. Write and balance the chemical equation.
2. Write the needed mole ratio between reactants.
3. What is the mole ratio you have from the data?
4. What is in excess and what is limited? (Show Work)
5. What is the theoretical yield of Aluminum Chloride?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Questions

Biology, 10.04.2020 15:48



Mathematics, 10.04.2020 15:48


Mathematics, 10.04.2020 15:48

Mathematics, 10.04.2020 15:48

Mathematics, 10.04.2020 15:48


Mathematics, 10.04.2020 15:48



Social Studies, 10.04.2020 15:48





Mathematics, 10.04.2020 15:48

Mathematics, 10.04.2020 15:48

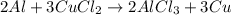
 : 2 moles of
: 2 moles of 
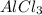 is 29.3 g
is 29.3 g 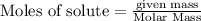
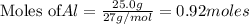
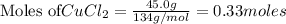
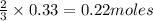 of
of