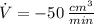Chemistry, 01.04.2021 17:40 jeronimo18
Boyle's Law states that when a sample of gas is compressed at a constant temperature, the pressure P and volume V satisfy the equation PV = C, where C is a constant. Suppose that at a certain instant the volume is 300 cm3, the pressure is 180 kPa, and the pressure is increasing at a rate of 30 kPa/min. At what rate is the volume decreasing at this instant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Boyle's Law states that when a sample of gas is compressed at a constant temperature, the pressure P...
Questions


Mathematics, 30.04.2021 07:50

Mathematics, 30.04.2021 07:50


Mathematics, 30.04.2021 07:50


English, 30.04.2021 07:50

Mathematics, 30.04.2021 07:50




Mathematics, 30.04.2021 07:50








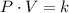 (1)
(1)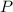 - Pressure, in kilopascals.
- Pressure, in kilopascals.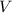 - Volume, in cubic centimeters.
- Volume, in cubic centimeters.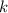 - Proportionality constant, in kilopascal-cubic centimeters.
- Proportionality constant, in kilopascal-cubic centimeters.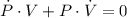 (2)
(2)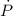 - Rate of change of the pressure, in kilopascals per minute.
- Rate of change of the pressure, in kilopascals per minute. - Rate of change of the volume, in cubic centimeters per minute.
- Rate of change of the volume, in cubic centimeters per minute.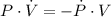
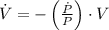
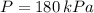 ,
, 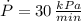 and
and 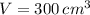 , then the rate of change of the volume is:
, then the rate of change of the volume is: