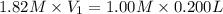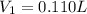A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring out some aqueous silver nitrate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the silver nitrate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring...
Questions




Mathematics, 20.06.2020 08:57



Mathematics, 20.06.2020 08:57

Mathematics, 20.06.2020 08:57

History, 20.06.2020 08:57



Mathematics, 20.06.2020 08:57

English, 20.06.2020 08:57

English, 20.06.2020 08:57

Mathematics, 20.06.2020 08:57




Mathematics, 20.06.2020 08:57

Mathematics, 20.06.2020 08:57

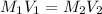
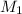 = molarity of stock silver nitrate solution = 1.82 M
= molarity of stock silver nitrate solution = 1.82 M
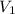 = volume of stock silver nitrate solution = ?
= volume of stock silver nitrate solution = ?