Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ...

Chemistry, 02.04.2021 01:40 adelawilliams60
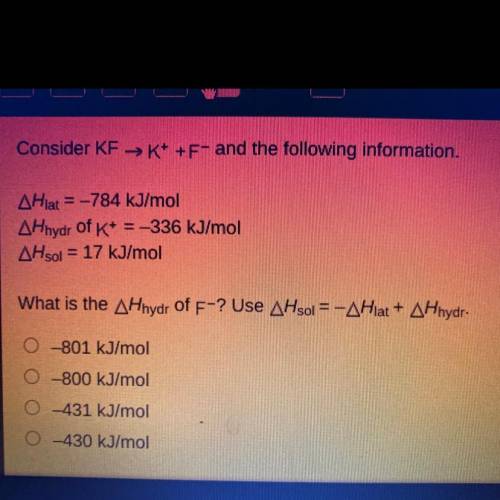
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ/mol
AHsol = 17 kJ/mol
What is the AHhydr of F-? Use AHsol = -AHlat + AHhydr.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


You know the right answer?
Questions

Computers and Technology, 27.11.2021 02:40


English, 27.11.2021 02:40

Mathematics, 27.11.2021 02:40

Mathematics, 27.11.2021 02:40





Mathematics, 27.11.2021 02:40


English, 27.11.2021 02:40

Mathematics, 27.11.2021 02:40

Biology, 27.11.2021 02:40





History, 27.11.2021 02:40

Mathematics, 27.11.2021 02:40



