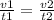Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3

Chemistry, 23.06.2019 15:00
What is the mass in grams of 0.94 moles of sodium bicarbonate, nahco3?
Answers: 1
You know the right answer?
At standard temperature, a gas has a volume of 275 mL. The temperature is then increased to
130. C,...
Questions

Mathematics, 04.03.2021 04:40


Mathematics, 04.03.2021 04:40



Chemistry, 04.03.2021 04:40

History, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40

Arts, 04.03.2021 04:40



Chemistry, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40


Mathematics, 04.03.2021 04:40



Mathematics, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40

Mathematics, 04.03.2021 04:40