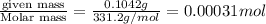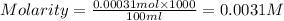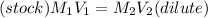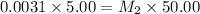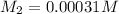Chemistry, 02.04.2021 03:00 kseniyayakimov
A stock solution was created by adding 0.1042 g of lead (II) nitrate to a 100.00 mL volumetric flask and diluting to volume with deionized water. A diluted solution was then created by removing 5.00 mL of the stock solution and placing into into a 50.00 mL volumetric flask and then diluting to volume with deionized water. What is the concentration (in molarity, M) of the diluted solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
A stock solution was created by adding 0.1042 g of lead (II) nitrate to a 100.00 mL volumetric flask...
Questions



Social Studies, 05.04.2020 18:26

Mathematics, 05.04.2020 18:26

Geography, 05.04.2020 18:26



Mathematics, 05.04.2020 18:26



Mathematics, 05.04.2020 18:26




History, 05.04.2020 18:27

Mathematics, 05.04.2020 18:27

Mathematics, 05.04.2020 18:27



Mathematics, 05.04.2020 18:28

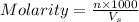
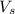 = volume of solution in ml
= volume of solution in ml
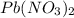 =
=