

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
You are given a stock solution of 500.0 mL of 1.00M magnesium chloride solution. Calculate the volum...
Questions


Mathematics, 07.01.2020 05:31



Mathematics, 07.01.2020 05:31

World Languages, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31





Mathematics, 07.01.2020 05:31






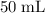 of the stock solution would be required.
of the stock solution would be required. 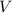 contains a solute with a concentration of
contains a solute with a concentration of  . The quantity
. The quantity 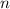 of that solute in this solution would be:
of that solute in this solution would be: 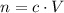 .
.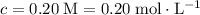 . The volume of this solution is
. The volume of this solution is 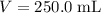 . Calculate the quantity of the solute (magnesium chloride) in the required solution:
. Calculate the quantity of the solute (magnesium chloride) in the required solution: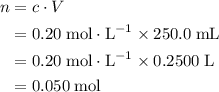 .
.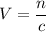 .
.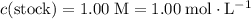 .
.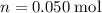 .
.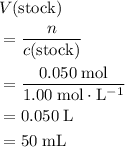 .
.

