
Chemistry, 02.04.2021 22:00 berlyntyler
Mass-Volume/Volume-Mass
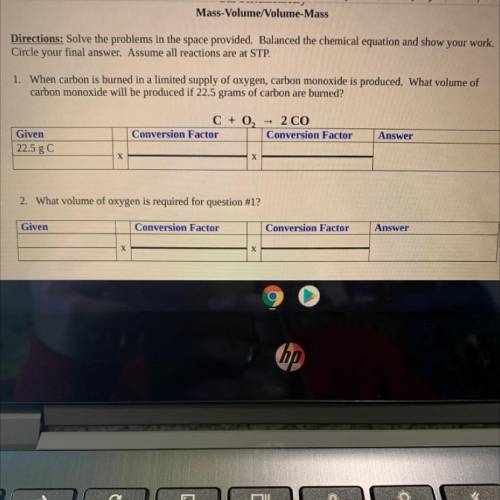
Directions: Solve the problems in the space provided. Balanced the chemical equation and show your work.
Circle your final answer. Assume all reactions are at STP.
1. When carbon is burned in a limited supply of oxygen, carbon monoxide is produced. What volume of
carbon monoxide will be
produced if 22.5 grams of carbon are burned?
C + O2 = 2 CO

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Mass-Volume/Volume-Mass
Directions: Solve the problems in the space provided. Balanced the chemica...
Questions


Biology, 29.08.2019 11:50

Physics, 29.08.2019 11:50






Health, 29.08.2019 11:50




Physics, 29.08.2019 11:50






English, 29.08.2019 11:50

Chemistry, 29.08.2019 11:50



