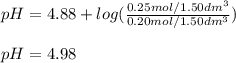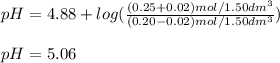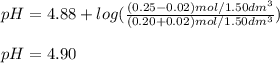A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate (CH3CH2COONa) in 1.50 dm3.
What is the pH of this buffer?
Enter your answer using two decimal places.
What is the pH of the buffer after the addition of 0.02 mol of NaOH?
What is the pH of the buffer after the addition of 0.02 mol of HI?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 08:00
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
You know the right answer?
A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate...
Questions


Computers and Technology, 18.10.2020 21:01

Biology, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01



Mathematics, 18.10.2020 21:01


Mathematics, 18.10.2020 21:01


English, 18.10.2020 21:01





History, 18.10.2020 21:01



Mathematics, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01

![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/1238/6169/33848.png)