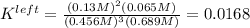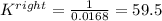Chemistry, 05.04.2021 03:50 addisynshepherd
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine the value of the equilibrium constant if the concentration of product C at equilibrium was measured to be 0.456 M.
Initial Conditions: No reactants present, [C] = 0.651 M, [D] = 0.754 M.
Equilibrium Conditions: [A] = ?, [Y] = ?, [C] = 0.456 M, [D] = ?.
A. K = 59.5
B. K = 37.2
C. K = 0.0269
D. K = 0.0168

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1
You know the right answer?
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine...
Questions



Computers and Technology, 13.12.2019 23:31






Computers and Technology, 13.12.2019 23:31











![K^{left}=\frac{[A]^2[Y]}{[C]^3[D]}](/tpl/images/1239/1292/6d673.png)
![[A]=2x](/tpl/images/1239/1292/d41d8.png)
![[Y]=x](/tpl/images/1239/1292/d76b1.png)
![[C]=0.651M-3x](/tpl/images/1239/1292/474d4.png)
![[D]=0.754M-x](/tpl/images/1239/1292/68d18.png)
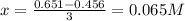
![[A]=2(0.065M)=0.13M](/tpl/images/1239/1292/b6025.png)
![[Y]=0.065M](/tpl/images/1239/1292/afc74.png)
![[D]=0.754M-0.065M=0.689M](/tpl/images/1239/1292/0e883.png)