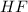Hydrogen fluoride reacts with ammonia in an acid-base reaction:
HF (aq) + NH3 (aq) -->
<...

Chemistry, 05.04.2021 07:50 SearchLight
Hydrogen fluoride reacts with ammonia in an acid-base reaction:
HF (aq) + NH3 (aq) -->
a. Determine the products made by completing the reaction above.
b. Identify and label one of the conjugate acid-base pairs.
Please I will give brainliest I’m stuck

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
Questions














Computers and Technology, 02.08.2019 18:10





Mathematics, 02.08.2019 18:10

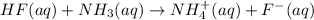
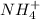 : conjugate acid of
: conjugate acid of 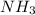
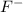 : conjugate base of
: conjugate base of