
Chemistry, 05.04.2021 18:10 coollid876
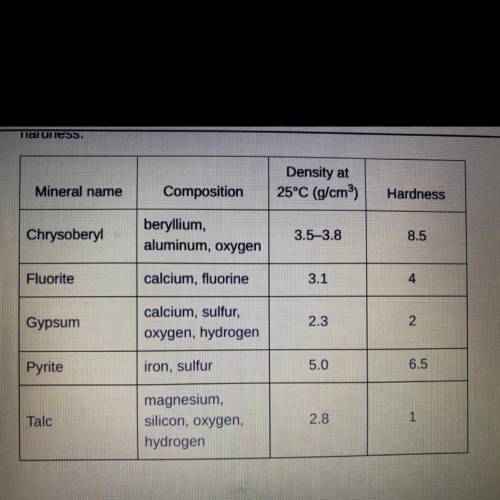
A student claimed that a sample of pyrite at 25°C with a volume of 10 cm3 would
have a mass of 2 g. Using the explanation of density given in the passage, explain
how the student incorrectly calculated the mass of the sample of pyrite. Then,
determine the actual mass of the 10 cm sample of pyrite.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
A student claimed that a sample of pyrite at 25°C with a volume of 10 cm3 would
have a mass of 2 g....
Questions

English, 26.09.2019 05:10



English, 26.09.2019 05:10

English, 26.09.2019 05:10

English, 26.09.2019 05:10


Mathematics, 26.09.2019 05:10






Business, 26.09.2019 05:10

Mathematics, 26.09.2019 05:10


Biology, 26.09.2019 05:10

Mathematics, 26.09.2019 05:10

Mathematics, 26.09.2019 05:10



