
Chemistry, 05.04.2021 19:40 softballgirl3589
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0°C, and a pressure of 0.980 atm, and a mass of 0.207 g.
(hint: find moles first and remember that molar mass is the mass per mole”)
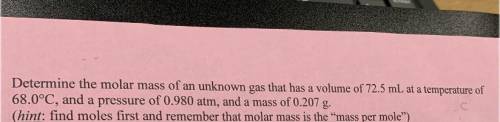

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0...
Questions

Mathematics, 03.08.2019 00:30




English, 03.08.2019 00:30


Mathematics, 03.08.2019 00:30

History, 03.08.2019 00:30

Mathematics, 03.08.2019 00:30

History, 03.08.2019 00:30

Mathematics, 03.08.2019 00:30


Mathematics, 03.08.2019 00:30



Mathematics, 03.08.2019 00:30

History, 03.08.2019 00:30





