
Chemistry, 06.04.2021 21:00 mamdouh2893
A buffer is made with 0.493 M of the weak base methylamine (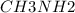 ) with 0.493 M
) with 0.493 M 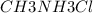 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?
If 0.100 M HBr is added to the buffer, what is the pH?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions

Mathematics, 03.03.2021 15:50


Physics, 03.03.2021 15:50

Mathematics, 03.03.2021 15:50

Mathematics, 03.03.2021 15:50

Mathematics, 03.03.2021 15:50

Advanced Placement (AP), 03.03.2021 15:50



Mathematics, 03.03.2021 15:50


Mathematics, 03.03.2021 15:50

Law, 03.03.2021 15:50

English, 03.03.2021 15:50



Mathematics, 03.03.2021 15:50



Computers and Technology, 03.03.2021 15:50



