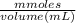Chemistry, 04.02.2020 14:50 snowprincess99447
A30.0-ml sample of 0.165 m propanoic acid is titrated with 0.300 m koh.
1. calculate the ph at 5 ml of added base.
2. calculate the ph at one-half of the equivalence point. (equivalence point is 8.96)
3. calculate the ph at 20 ml of added base.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
A30.0-ml sample of 0.165 m propanoic acid is titrated with 0.300 m koh.
1. calculate th...
1. calculate th...
Questions

Health, 08.10.2020 01:01


History, 08.10.2020 01:01


Mathematics, 08.10.2020 01:01

Geography, 08.10.2020 01:01



Social Studies, 08.10.2020 01:01


Arts, 08.10.2020 01:01




History, 08.10.2020 01:01


Arts, 08.10.2020 01:01

Biology, 08.10.2020 01:01