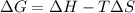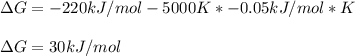What is the value for AG at 5000 K if AH = -220 kJ/mol and S= -0.05 kJ/(mol-K)?
A. -195 kJ
B....

Chemistry, 07.04.2021 08:40 julieariscar769
What is the value for AG at 5000 K if AH = -220 kJ/mol and S= -0.05 kJ/(mol-K)?
A. -195 kJ
B. -470. kJ
C. 30 kJ
D. 470 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Questions

Mathematics, 27.04.2021 20:50

History, 27.04.2021 20:50

Mathematics, 27.04.2021 20:50



Social Studies, 27.04.2021 20:50

English, 27.04.2021 20:50


Mathematics, 27.04.2021 20:50






Mathematics, 27.04.2021 20:50