
Chemistry, 07.04.2021 19:50 nyasiasaunders1234
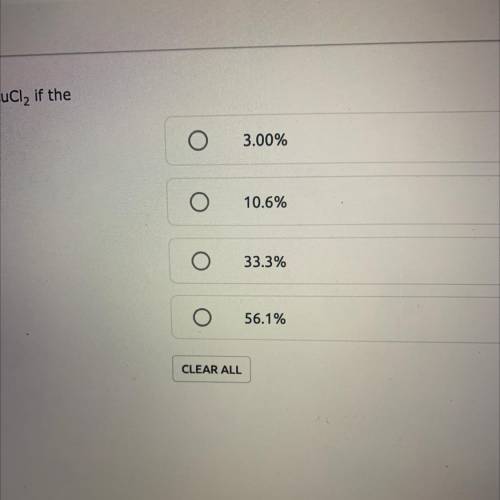
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield is 18.81g? % Yield = (Actual Yield/Theoretical Yield) x 100

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield...
Questions

Mathematics, 10.07.2019 13:50







Social Studies, 10.07.2019 13:50



Mathematics, 10.07.2019 13:50


Mathematics, 10.07.2019 13:50


Mathematics, 10.07.2019 13:50



Mathematics, 10.07.2019 13:50

Social Studies, 10.07.2019 13:50



