
Chemistry, 22.09.2019 17:30 devbar3416
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water that results in a decrease in the water’s temperature. which of the following potential energy diagrams best illustrates the energy change of this dissolving process?


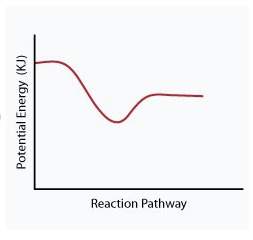
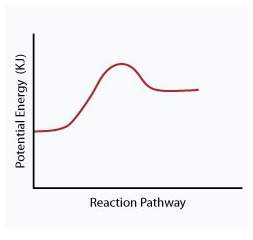
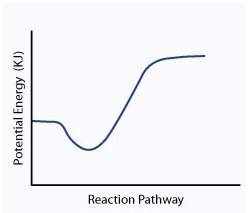

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water t...
Questions

History, 19.03.2021 01:00

Computers and Technology, 19.03.2021 01:00

Biology, 19.03.2021 01:00




History, 19.03.2021 01:00


Mathematics, 19.03.2021 01:00


History, 19.03.2021 01:00


Social Studies, 19.03.2021 01:00


Mathematics, 19.03.2021 01:00



Mathematics, 19.03.2021 01:00


Biology, 19.03.2021 01:00



