
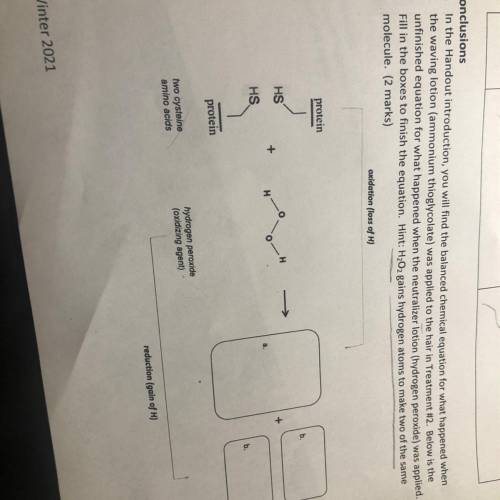
Conclusions
1. In the Handout introduction, you will find the balanced chemical equation for what happened when
the waving lotion (ammonium thioglycolate) was applied to the hair in Treatment #2. Below is the
unfinished equation for what happened when the neutralizer lotion (hydrogen peroxide) was applied.
Fill in the boxes to finish the equation. Hint: H2O2 gains hydrogen atoms to make two of the same
molecule. (2 marks)
E
oxidation (loss of H)
protein
b.
HS
+
HS
:
b.
protein
two cysteine
amino acids
hydrogen peroxide
(oxidizing agent)
reduction (gain of H)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Conclusions
1. In the Handout introduction, you will find the balanced chemical equation for what h...
Questions



History, 08.04.2021 08:50

Social Studies, 08.04.2021 08:50

Mathematics, 08.04.2021 08:50

Mathematics, 08.04.2021 08:50

Health, 08.04.2021 08:50



Mathematics, 08.04.2021 08:50


Mathematics, 08.04.2021 08:50

History, 08.04.2021 08:50

Mathematics, 08.04.2021 08:50








