
Chemistry, 08.04.2021 01:20 emmaja121003
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
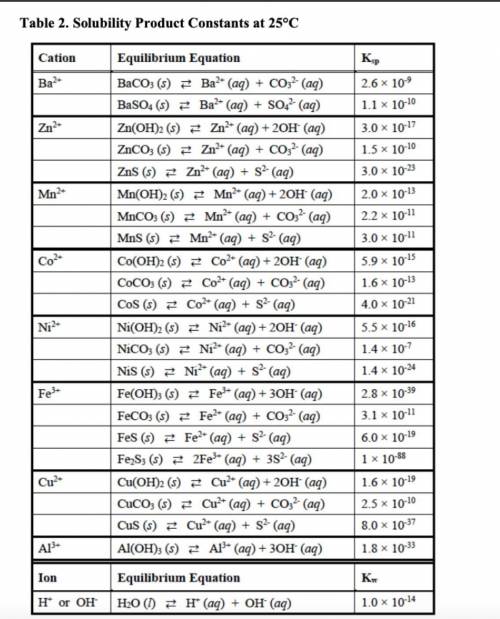
out the corresponding solubility equilibrium equations that would give rise to a precipitate with ammonia. Be careful to use the appropriate arrows (⇄ vs. →), depending on the compound’s solubility. Using the Ksp values provided in Table 2, predict whether it would form using 5 drops (0.25 mL) of 3 M NH3 and 1 mL of 0.10 M metal nitrate solution.
Your answer must include:
• The relevant chemical equation(s) showing dissociation or equilibrium of the
products into their ionic components.
• The Ksp values and equations
• Calculations showing how concentrations were determined
• Unique Qsp calculations
• A comparison of Qsp and Ksp, and discussion of what this comparison predicts
about precipitate (solid) formation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
out the corresp...
Questions


Mathematics, 17.11.2021 16:30


English, 17.11.2021 16:30


Mathematics, 17.11.2021 16:30



Computers and Technology, 17.11.2021 16:30

SAT, 17.11.2021 16:30


Biology, 17.11.2021 16:30



Mathematics, 17.11.2021 16:30

Mathematics, 17.11.2021 16:40

Mathematics, 17.11.2021 16:40


Mathematics, 17.11.2021 16:40

English, 17.11.2021 16:40



