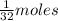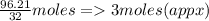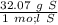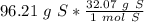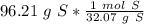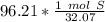Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
6) A student measures out 96.21 g of sulfur for an experiment. How many moles of Sulfur are in this...
Questions


Mathematics, 12.11.2020 04:00


Mathematics, 12.11.2020 04:00





Mathematics, 12.11.2020 04:00


Mathematics, 12.11.2020 04:00

Social Studies, 12.11.2020 04:00

Mathematics, 12.11.2020 04:00

Mathematics, 12.11.2020 04:00




Mathematics, 12.11.2020 04:00

Health, 12.11.2020 04:00