Calculate the number of moles or atoms in each of
the following quantities.
a. moles of...

Chemistry, 08.04.2021 18:20 greystokey
Calculate the number of moles or atoms in each of
the following quantities.
a. moles of each element in 3.35 moles of aspirin
(C9H8O4)
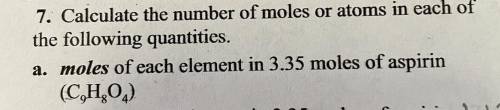

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Questions

Mathematics, 24.09.2020 01:01


Mathematics, 24.09.2020 01:01

Biology, 24.09.2020 01:01

Mathematics, 24.09.2020 01:01

History, 24.09.2020 01:01

Mathematics, 24.09.2020 01:01


Mathematics, 24.09.2020 01:01

Mathematics, 24.09.2020 01:01


Computers and Technology, 24.09.2020 01:01




Chemistry, 24.09.2020 01:01




Mathematics, 24.09.2020 01:01




