
A 5.0 g sample of a pesticide was decomposed with metallic sodium in alcohol, and the liberated chloride ion was precipitated with AgCl. Express the results of this analysis in terms of percent DDT (C14H9Cl5, molar mass, 354.72 g/mol)) based on the recovery of 0.1606 g of AgCl

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
A 5.0 g sample of a pesticide was decomposed with metallic sodium in alcohol, and the liberated chlo...
Questions

Mathematics, 25.02.2020 19:57


Mathematics, 25.02.2020 19:58






History, 25.02.2020 19:58









Chemistry, 25.02.2020 19:58

English, 25.02.2020 19:58

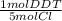 = 2.24x10⁻⁴ mol DDT
= 2.24x10⁻⁴ mol DDT

