
Chemistry, 08.04.2021 22:20 cornpops4037
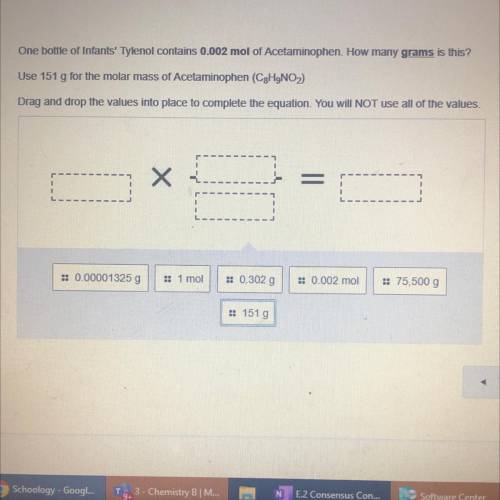
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151 g for the molar mass of Acetaminophen (C2H9NO)
Drag and drop the values into place to complete the equation. You will NOT use all of the values.
Х
= 0.00001325 g
1 moi
:: 0.302 g
: 0.002 mol
= 75,500 g
:: 151 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151...
Questions



Biology, 13.10.2019 15:50

Physics, 13.10.2019 15:50




Mathematics, 13.10.2019 15:50

Chemistry, 13.10.2019 15:50


History, 13.10.2019 15:50


English, 13.10.2019 15:50

Social Studies, 13.10.2019 15:50



Social Studies, 13.10.2019 15:50





