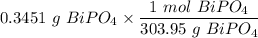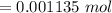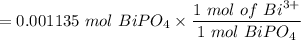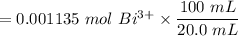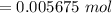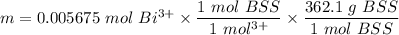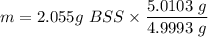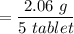Chemistry, 08.04.2021 22:20 adayisenga
The tablets were crushed, and 4.9993 g of the powder was transferred to a beaker and reacted with HCl. After filtration, the filtrate was transferred to a 100-mL volumetric flask and diluted with water. 20.00 mL of this stock solution were combined with 0.2 M Na3PO4. The resulting precipitate weighed 0.3451 g after drying. Calculate the moles of BiPO4 precipitated, the moles of Bi3 in the stock solution, and the mass of BSS per tablet.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
The tablets were crushed, and 4.9993 g of the powder was transferred to a beaker and reacted with HC...
Questions

Biology, 30.07.2019 14:30

History, 30.07.2019 14:30


History, 30.07.2019 14:30

Mathematics, 30.07.2019 14:30







History, 30.07.2019 14:30

Business, 30.07.2019 14:30



History, 30.07.2019 14:30

Social Studies, 30.07.2019 14:30



Social Studies, 30.07.2019 14:30