
Chemistry, 09.04.2021 03:50 whitethunder05
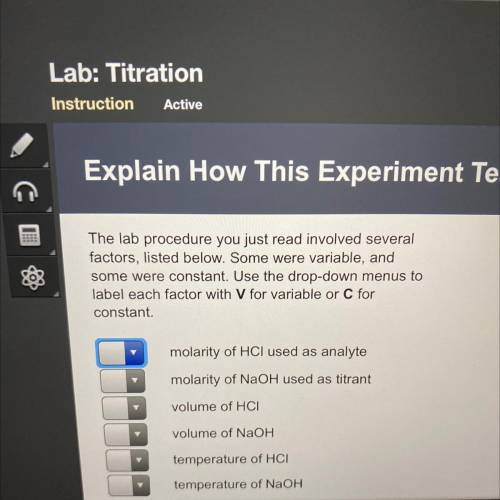
The lab procedure you just read involved several
factors, listed below. Some were variable, and
some were constant. Use the drop-down menus to
label each factor with V for variable or C for
constant.
molarity of HCl used as analyte
molarity of NaOH used as titrant
volume of HCI
volume of NaOH
temperature of HCI
temperature of NaOH

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
The lab procedure you just read involved several
factors, listed below. Some were variable, and
Questions


Biology, 15.04.2021 15:10



Mathematics, 15.04.2021 15:10




Computers and Technology, 15.04.2021 15:10

Mathematics, 15.04.2021 15:10

Chemistry, 15.04.2021 15:10

Mathematics, 15.04.2021 15:10



Mathematics, 15.04.2021 15:10




Business, 15.04.2021 15:10



