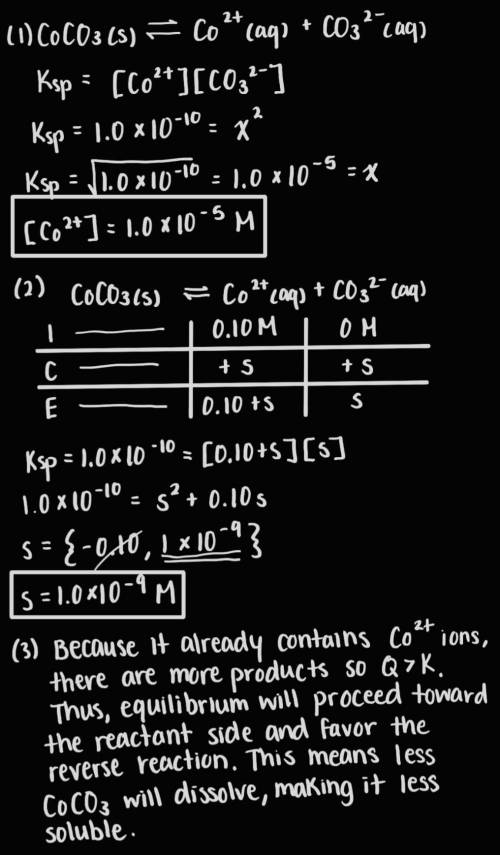Chemistry, 09.04.2021 23:50 valoiserika1229
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1.0 × 10^−10.
A. Calculate the value of [Co2+] in a saturated solution of CoCO3 in distilled water.
B. If 0.10 M of Co2+ is already present in distilled water, calculate the molar solubility of CoCO3(s).
C. Explain why CoCO3 is less soluble in distilled water that already contains Co2+

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1....
Questions

Biology, 02.10.2021 09:10

Social Studies, 02.10.2021 09:10

Mathematics, 02.10.2021 09:10

Chemistry, 02.10.2021 09:10



Mathematics, 02.10.2021 09:10


Mathematics, 02.10.2021 09:10



Geography, 02.10.2021 09:10

Arts, 02.10.2021 09:10

History, 02.10.2021 09:10

SAT, 02.10.2021 09:10

Social Studies, 02.10.2021 09:10

History, 02.10.2021 09:10


Mathematics, 02.10.2021 09:10

Arts, 02.10.2021 09:10

![K = \frac{[products]}{[reactants]}](/tpl/images/1250/0658/0c10f.png) increases.
increases.