
Chemistry, 10.04.2021 01:10 mendezmarco2004
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds as completely as possible. Which TWO of the following statements are correct? *
A. CO(g) is the limiting reactant.
B. O2(g) is the limiting reactant.
C. 4mol of CO(g) reacts.
D. 2mol of CO(g) remains unreacted.
E. 144g of CO2(g) is produced.
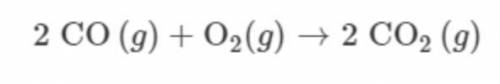

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds...
Questions

Mathematics, 10.09.2020 01:01

History, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

History, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01





Social Studies, 10.09.2020 01:01




Computers and Technology, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01





