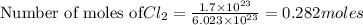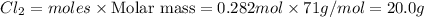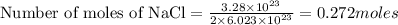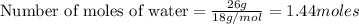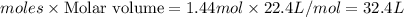1. How many grams are in 1.7 x 10^23 particles of Cl2?
2. How many moles are in 3.28 x 10^23 atoms of NaCl? *
3. If I were to determine how many liters 26 grams of water is, what type of conversion would this be? *
A Mass --> Moles --> Particles
B Mass --> Moles --> Volume
C Volume --> Mass --> Moles
D Moles --> Mass --> Volume

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2


Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
1. How many grams are in 1.7 x 10^23 particles of Cl2?
2. How many moles are in 3.28 x 10^23 atoms...
Questions

English, 10.06.2021 16:50


Physics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Business, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Arts, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

World Languages, 10.06.2021 16:50


Biology, 10.06.2021 16:50



Mathematics, 10.06.2021 16:50

Mathematics, 10.06.2021 16:50

Biology, 10.06.2021 16:50

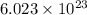 of particles.
of particles.
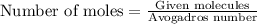 or
or 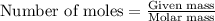 or
or