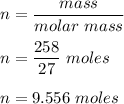Chemistry, 11.04.2021 03:20 mstrish71oteauw
6 NaOH + 2 Al --> 2 Na3AlO3 + 3 H2 If you start with 258 g of Al, how many moles of Na3AlO3 can you make?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
6 NaOH + 2 Al --> 2 Na3AlO3 + 3 H2 If you start with 258 g of Al, how many moles of Na3AlO3 can y...
Questions











Mathematics, 10.11.2020 16:40




Mathematics, 10.11.2020 16:40

Physics, 10.11.2020 16:40


Mathematics, 10.11.2020 16:40

Mathematics, 10.11.2020 16:40

 .
.