B. 1.333 x 10^23 atoms of B

Chemistry, 11.04.2021 05:10 maddiehope6893
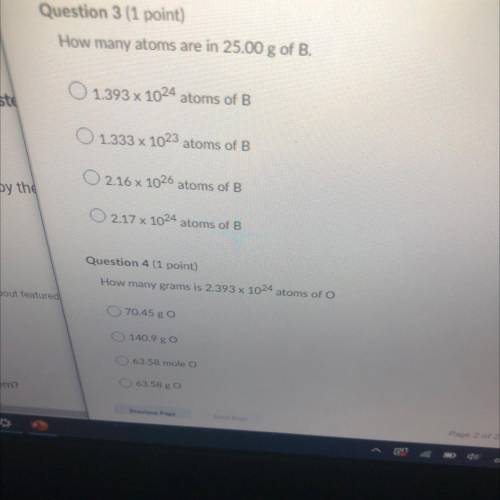
How many atoms are in 25.00 g of B?
A. 1.393 x 10^24 atoms of B
B. 1.333 x 10^23 atoms of B
C. 2.16 x 10^26 atoms of B
D.217 x 10^24 atoms of B
How many grams is 2.393 x 10^24 atoms of O?
A. 70.45 g O
B. 140.9 g O
C. 63.58 mole O
D. 63.58 g O
Please answer both questions if you can, thanks for all the efforts :)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1



Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
How many atoms are in 25.00 g of B?
A. 1.393 x 10^24 atoms of B
B. 1.333 x 10^23 atoms of B
B. 1.333 x 10^23 atoms of B
Questions


Mathematics, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31


History, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31





History, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31

History, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31

Mathematics, 23.12.2019 01:31

Chemistry, 23.12.2019 01:31



Mathematics, 23.12.2019 02:31



