
Chemistry, 11.04.2021 08:40 susannaking5852
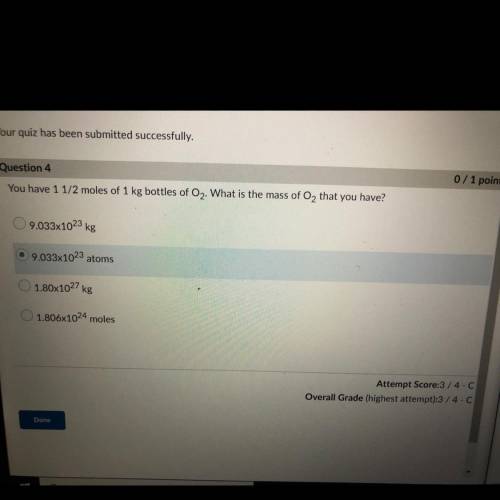
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg
B. 9.033x10^23 atoms
C. 1.80x10^27 kg
D. 1.806x10^24 moles
It is not B as I have already tried it :(

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

You know the right answer?
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg...
Questions






Mathematics, 03.06.2021 16:00










Mathematics, 03.06.2021 16:00

Health, 03.06.2021 16:00

Mathematics, 03.06.2021 16:00

Mathematics, 03.06.2021 16:00

Chemistry, 03.06.2021 16:00



