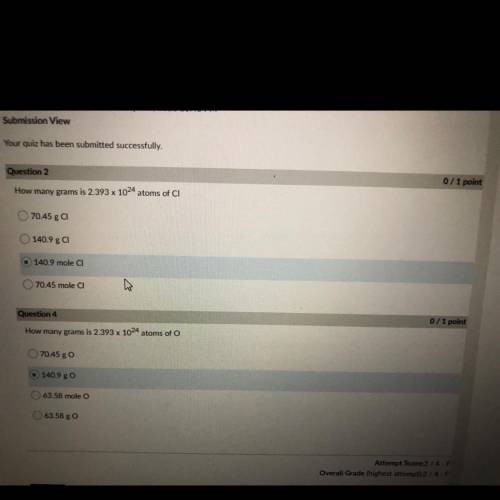
How many grams is 2.393 x 10^24 atoms of CI
70.45 g CI
140.9 ga
140.9 mole CI
70....


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

You know the right answer?
Questions

History, 28.07.2019 18:00


History, 28.07.2019 18:00

History, 28.07.2019 18:00

History, 28.07.2019 18:00

Biology, 28.07.2019 18:00

History, 28.07.2019 18:00

Chemistry, 28.07.2019 18:00







Business, 28.07.2019 18:00

Biology, 28.07.2019 18:00


Biology, 28.07.2019 18:00